Matter is everything that you can see touch and feel. It takes up space, has mass, and can be dead or alive. The forms of matter are SOLID, LIQUID, GAS and PLASMA All matter is made of particles called ATOMS
You are watching: Matter: Solids, Liquids, and Gases
ATOMS – “ATOMA” means indivisible. PROTONS and NEUTRONS form the NUCLEUS. ELECTRONS spin around the nucleus. Atoms differ only in the # of protons in the nucleus and # of electrons spinning around it. This is what determines whether it is a solid, liquid, or gas. Hydrogen is the lightest element. Uranium is the heaviest. Molecules are made of 2 or more atoms and are always in motion. Compounds are atoms joined together of 2 or more kinds of atoms = H2O
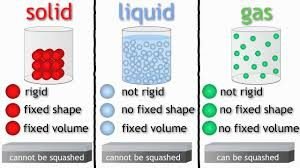
CHARACTERISTICS OF A SOLID:
1. molecules are very close together and jiggle in place 2. Keep a definite shape and size 3. Most molecules are arranged in patterns like crystals 4. They change when a force acts upon them 5. They have physical the properties of color, texture, hardness, odor, luster, melting & freezing points, malleability, and ductility.
Mass is the amount of matter an object contains. / The amount of force to move a weight is the gravitational force and depends on the distance from gravity. If you weigh 100 on earth you would weigh 16 lbs. on the moon but the mass remains the same. Find the center of gravity with a straw.
CHARACTERISTICS OF LIQUIDS:
1. The atoms are farther apart and slide around each other allowing flow. 2. Have no definite shape but moves freely, but have a definite size. 3. Exhibit capillary action. Water is pulled into spaces uphill as molecules exhibit cohesion (molecules have a strong attraction for each other) Water moves from soil into roots by the method of cohesion. 4. Has surface tension (float a needle or paper clip on water) 5. Some liquids are lighter and less dense than others.
Natural Liquids are water, petroleum, blood, lava, sap, and honey. You can layer liquids. Oil dissolves in alcohol.
CHARACTERISTICS OF GAS:
Read more : Beverly Ann Reynolds Riggs
1. Molecules are far apart moving fast and bouncing off one another 2. Has no definite shape but moves freely 3. Flows through neon signs 4. Expands when heated 5. INVISIBLE 6. Most are odorless 7. It contracts when cooled

When matter expands it gets bigger and when it contracts it “usually” gets smaller. Matter usually contracts when cooled and expands when heated. Water is one example of a liquid than expands and gets larger when frozen. Hoses can be ruined if water left in them freezes or water pipes may freeze and burst. A Thermometer is often used to measure the temperature of the home or food when cooking. The temperature is also a gage for when to plant.
An ELEMENT consists of ONE KIND OF ATOM. MANDELEV created the periodic table of elements. BOYL identified 100 kinds of elements. There have been a few more discovered recently. Mass = bulk or quantity. Adhesion is when the molecules are attracted to surface molecules of other substances as water clings to the teeth of a comb.
Water is the only element found in all three states in nature. Gas as fog, clouds, steam, mist Solid as arctic ice, hail, icicles, sleet, snow, frost Liquid as rain, ocean, dew, rivers, ponds, ground water and puddles.
We use all three states of water in everyday life. Living things are mostly made of water!
Solid + heat = liquid . . . liquid + cold = solid liquid + heat = gas
CO2 under pressure and then cooled turns the gas to dry ice. Dry ice melts to a gas. SUBLIMATION is when a solid is turned to gas
If warm air is cooled or meets cold air it forms condensation. Different liquids evaporate into a gas at different rates (paint alcohol, water, and vinegar on a chalkboard) Epsom salts and water, plus add a little ammonia, and it turns white forming a precipitate. Teach the water cycle song about Evaporation, Condensation, and Precipitation
Experiments: 1. CO2 from borax and vinegar to blow up balloon 2. Two liquids combine to a solid in slime 3. Thermometer goes up from heat 4. Teach them the Molecule dance 5. Water song 6. Capillary action with different kinds of paper strips 7. Layer liquids
Plasma is an electrically neutral medium of unbound positive and negative particles ( the overall charge of a plasma is roughly zero). Although they are unbound, these particles are not ‘free’ in the sense of not experiencing forces. When the charges move, they generate electric currents with magnetic fields and they are affected by each other’s fields. This governs their collective behavior with many degrees of freedom. A definition can have three criteria:
The plasma approximation: Charged particles must be close enough together that each particle influences many nearby charged particles, rather than just interacting with the closest particle (these collective effects are a distinguishing feature of a plasma). The plasma approximation is valid when the number of charge carriers within the sphere of influence (called the Debye sphere whose radius is the Debye screening length) of a particular particle is higher than unity to provide collective behavior of the charged particles. The average number of particles in the Debye sphere is given by the plasma parameter.
Bulk interactions: The Debye screening length is short compared to the physical size of the plasma. This criterion means that interactions in the bulk of the plasma are more important than those at its edges, where boundary effects may take place. When this criterion is satisfied, the plasma is quasineutral.
Plasma frequency: The electron plasma frequency (measuring plasma oscillations of the electrons) is large compared to the electron-neutral collision frequency (measuring frequency of collisions between electrons and neutral particles). When this condition is valid, electrostatic interactions dominate over the processes of ordinary gas kinetics.
Ranges of parameters Plasma parameters can take on values varying by many orders of magnitude, but the properties of plasmas with apparently disparate parameters may be very similar. The following chart considers only conventional atomic plasmas and not exotic phenomena like quark gluon plasmas:
Terrestrial Plasma:
Lightning St. Elmo’s fire Upper-atmospheric lightning (Blue jets, Blue starters, Gigantic jets, ELVES) Sprites The ionosphere The plasmasphere The polar aurorae Some flames The polar wind, a plasma fountain
Celestial plasma:
The Sun and other stars (plasmas heated by nuclear fusion) The solar wind The interplanetary medium (space between planets) The interstellar medium (space between star systems) The Intergalactic medium (space between galaxies) The Io-Jupiter flux tube Accretion discs Interstellar nebulae Cometary ion tail
Source: https://gardencourte.com
Categories: Garden news

